Description
Mercury sulfite electrode (Hg/Hg2SO4) is used as a reference element in acid meters, ion meters, potentiometric titrators and electrochemical workstations. It can be used as a reference electrode for trace chlorine determination in conjunction with various ion-selective electrodes and metal electrodes, and can be applied to potentiometric measurements in acidic solutions.
| Model No. | Electrolyte Solution | Remarks |
| Hg2SO4 Electrode | Saturated Potassium Sulfate solution (K2SO4 ) | Standard |
Technical Specifications

User Manual, Maintenance and Precautions
- Remove the rubber cap from the liquid junction before using the mercury sulfate electrode.
- Ensure the salt bridge of the mercury sulfate electrode is filled with the salt bridge solution during measurement to complete the circuit. The solution level in the salt bridge should be higher than the sample solution to prevent reverse contamination.
- The salt bridge solution must be free of large air bubbles to maintain an unobstructed electronic measurement circuit. If bubbles are present, hold the electrode firmly and shake it vigorously or tap it upright to release the bubbles.
- Do not use the mercury sulfate electrode in media that react with it. If measurements with this electrode are unavoidable, it is recommended that a salt bridge be added to the electrode to block the effect of the test solution on the core of the mercury sulfate electrode.
- Regularly clean and replace the salt bridge solution. For general cleaning, remove any adhering stains promptly. To replace the salt bridge solution, remove the rubber cap from the injection port at the top of the electrode, extract the old solution, and inject fresh solution.
- As the electrodes contain toxic substances such as mercury, strictly adhere to laboratory safety regulations during handling.
- Avoid prolonged exposure of the electrode to air (more than a few minutes) to prevent the solution in the glass tube from leaking and evaporating, which could impair performance. For short-term storage, immerse the electrode in K2SO4 solution of the corresponding concentration. For long-term storage, replace the salt bridge solution, seal the electrode, and store it in a dark place.
- When removing the electrode cap, it is recommended to remove the top and bottom electrode caps at the same time, so as not to cause a difference in air pressure between the inside and outside, which may cause the liquidus flow rate too fast.
- The recommended operating temperature for electrodes is room temperature, not exceeding 40°C.
- Reference electrodes, including mercury sulfate electrode, should not be cleaned using ultrasonic methods.
- The salt bridge solution for the mercury sulphite electrode is saturated K2SO4 and can be changed to other concentrations of K2SO4 solution. However, the electrode potential will also change due to the change in concentration and composition of the salt bridge solution, which may also affect the service life of the electrode.
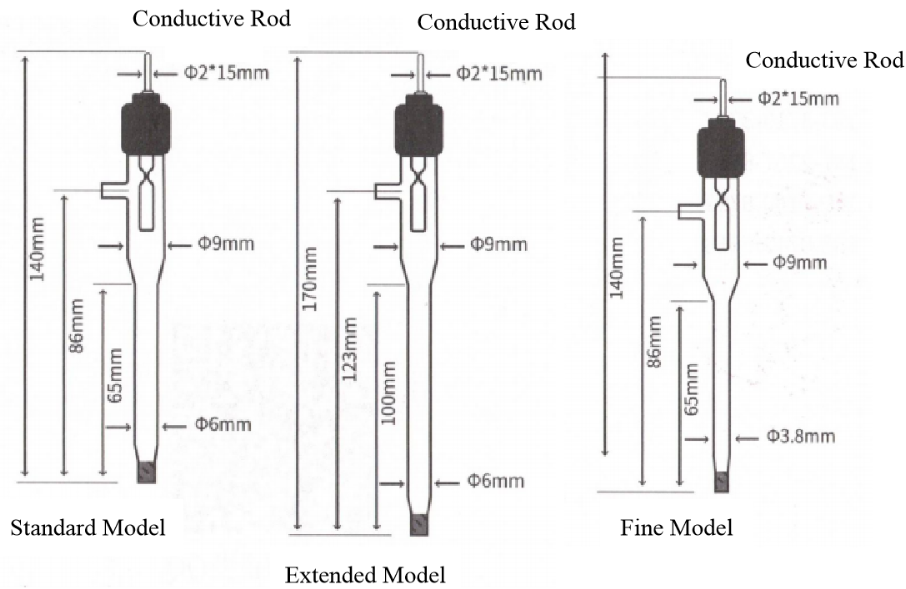
Product Illustration





Reviews
There are no reviews yet.